views
By proton number
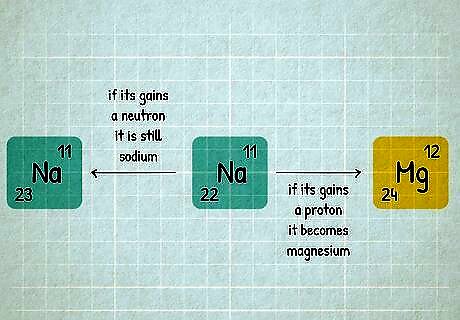
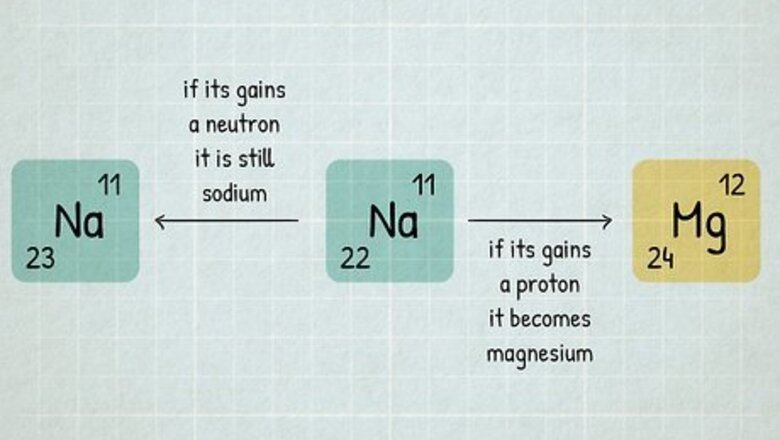
An element is defined by the number of protons in one atom. For example, every single atom of hydrogen has exactly one proton. We say that hydrogen has a proton number or atomic number of 1. The periodic table is arranged in order of proton number, which is why hydrogen is in the very first box with a 1 next to it. Atomic number is abbreviated "Z". If your homework says an element has Z=13, you can look for atomic number 13 on the periodic table and identify it as aluminum (Al). An atom can gain or lose neutrons and still be the same element. For instance, 11 22 N a {\displaystyle _{11}^{22}Na} {\displaystyle _{11}^{22}Na} is a sodium atom with 11 protons and 22 neutrons. If it gains a neutron, it is still sodium and becomes 11 23 N a {\displaystyle _{11}^{23}Na} {\displaystyle _{11}^{23}Na} (with 23 neutrons). But if you add a proton, it transforms from sodium to magnesium, 12 M g {\displaystyle _{12}Mg} {\displaystyle _{12}Mg}.
By electron count
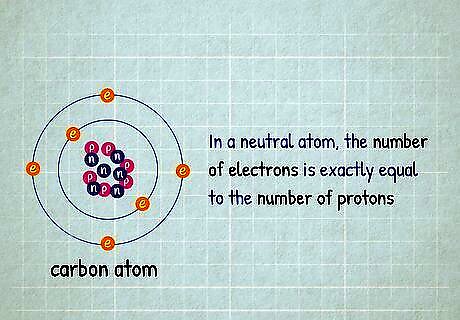
The total electron count equals the atomic number. In a neutral atom, the number of electrons is exactly equal to the number of protons. This number is the atomic number of the element, which you can look up on the periodic table. If you are a little further in your chemistry studies, you might be given an electron configuration to read. All of the superscript numbers () are electron counts, so add all these together to find the total number of electrons. For example, if you are asked which element has 8 electrons, look for the element with atomic number 8: oxygen. For a more advanced example, the configuration 1 s 2 2 s 2 2 p 2 {\displaystyle 1s^{2}2s^{2}2p^{2}} {\displaystyle 1s^{2}2s^{2}2p^{2}} has 2 {\displaystyle ^{2}} {\displaystyle ^{2}} electrons in the 1s shell, 2 {\displaystyle ^{2}} {\displaystyle ^{2}} in the 2s shell, and 2 {\displaystyle ^{2}} {\displaystyle ^{2}} in the 2p shell, for a total of 2+2+2=6. This is carbon, with atomic number 6. Note that this only holds true when the atoms are in electrically neutral states, not ionized. But unless specified otherwise, this is the state we talk about when we discuss element characteristics.
By electron configuration with the periodic table
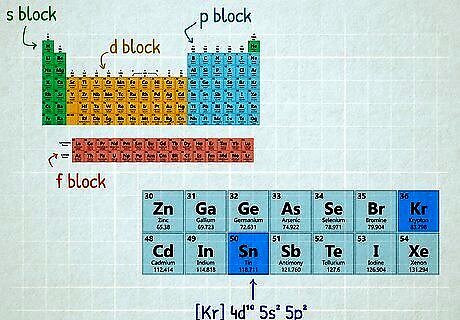
Memorize the periodic table structure to read electron configurations quickly. The structure of the periodic table is closely related to how electron orbitals are filled. With a little practice you can jump directly to the right region of the periodic table. Note that the electron configuration must be in its ground state for this to work. The first row (hydrogen and helium) fills up the 1s orbital from left to right. Think of these, plus all elements in the first two columns, as the "s-block". Each row of the "s-block" fills up one s orbital. The right-hand side of the table is the "p-block", starting with boron through neon. Each row of the "p-block" fills up one p orbital (starting with 2p). The transition metals in the center form the "d-block". Each row fills up one d orbital, starting with scandium through zinc filling 3d. The lanthanides and actinides at the bottom of the table fill the 4f and 5f orbitals. (Some elements here break the pattern, so double-check these.) For example, look at [ K r ] 5 s 2 4 d 10 5 p 2 {\displaystyle [Kr]5s^{2}4d^{10}5p^{2}} {\displaystyle [Kr]5s^{2}4d^{10}5p^{2}} and focus on the last orbital: 5 p 2 {\displaystyle 5p^{2}} {\displaystyle 5p^{2}}. Go to the "p-block" on the right, and count rows down from 2p (boron) until you reach 5p (indium). Since this element has two electrons in 5p, count two elements into this row of the p-block to get the answer: tin.
By spectroscopy

Compare the spectra to the known spectra of elements. In spectroscopy, scientists examine how light interacts with an unknown material. Each element releases a unique pattern of light, which you can see on the spectroscopy results, called "spectra". For example, a lithium spectrum has a very bright, thick green line, and several other fainter ones in different colors. If your spectrum has all those same lines on it, the light came from the element lithium. (Some types of spectra will show dark gaps instead of bright lines, but you can compare these the same way.) Want to know why this works? Electrons only absorb and emit light at very specific wavelengths (meaning specific colors). Different elements have different arrangements of electrons, which leads to different colors of bands. A more advanced spectroscope shows a detailed graph instead of a few lines. You can match the x-axis value at each peak to a table of known values to identify molecules. As you learn about different types of molecules, you'll learn to focus on just a few useful spots on the graph to save time.
By mass spectrum
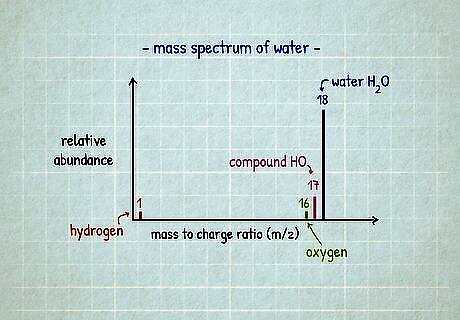
Look for elements whose atomic masses match the graph. A mass spectrometer sorts the components of a sample by mass. To read the bar graph showing the results, check the "m/z" axis for the values of the taller bars. Some values will match the atomic mass of an element that was part of the sample. Others (usually the larger ones) represent compounds, so that mass will equal the sum of masses of multiple atoms. Let's say the tallest bar is at m/z 18, with short bars at 1, 16, and 17. Only two of these match the atomic mass of an element: hydrogen (atomic mass 1) and oxygen (atomic mass 16). Adding these atoms together gives you the compounds HO (mass 1 + 16 = 17) and H2O (mass 1 + 1 + 16 = 18). This sample was water! Technically, a mass spectrometer ionizes the sample and sorts by the ratio of mass to charge (or m/z). But most ions will have a charge of 1, and so you can ignore the division problem and just look at mass. The smallest bars often represent small amounts of more charged particles that you can ignore for identification purposes.
















Comments
0 comment