Covid-19: Sputnik V Vaccine Makers Back to The Drawing Board For 2nd Time As Panel Demands More Data
views
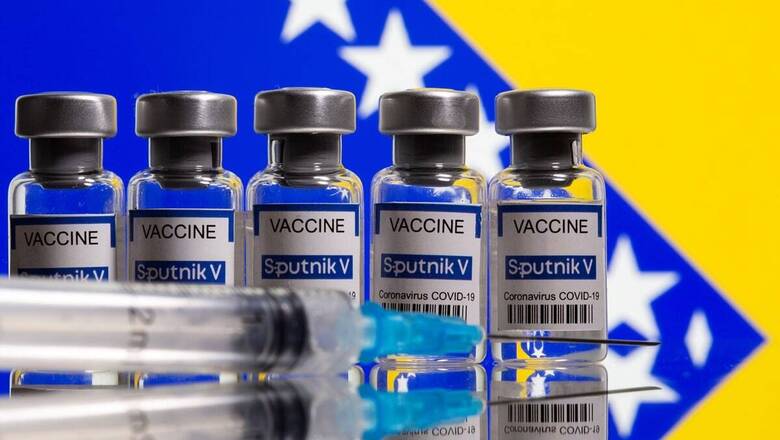
Russian COVID-19 vaccine Sputnik V was on Thursday denied emergency use authorisation for the second time by the Subject Expert Committee (SEC), constituted by the central government. The SEC met today on drugmaker Dr Reddy’s application seeking authorisation for the vaccine in India. The expert panel has asked Dr Reddy’s and the drugmaker to submit more data on the vaccine.
Meanwhile, Dr Reddy’s issued a statement saying, “We have had our meeting with the SEC today and will await the feedback from the CDSCO. We will provide an update once we have the feedback.”
Dr Reddy’s has partnered with the Russia Direct Investment Fund (RDIF) to bring the Sputnik V vaccine to India. The Ministry of Health had appointed the SEC to meet over Dr Reddy’s application on Wednesday. Dr Reddy’s has partnered with Russia Direct Investment Fund (RDIF) to bring the Sputnik V vaccine to India.
The Russian vaccine has shown efficacy of 91.6 %. Russia registered Sputnik V for public use in August, the first country to do so, though the approval came before the start of the large-scale trial in September.
Previously, Russia’s RDIF sovereign wealth fund has also announced four manufacturing deal with the Indian pharmaceutical makers Virchow Biotech Private Limited, Gland Pharma Pvt Ltd, Stelis Biopharma and Hetero Biopharma.
India has been pledged 125 million doses of the vaccine by Russia. India, the world’s largest vaccine maker, has become one of the biggest producers of the Sputnik V shot outside Russia. Other countries producing it include Brazil, China and South Korea.
The vaccination drive in India had started on January 16 with two vaccines that are home grown — Covaxin developed by Bharat Biotech and Oxford-AstraZeneca’s Covishield manufactured by the Serum Institute of India. Both the vaccines were granted “conditional approval” from the DCGI on January 3.
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.

















Comments
0 comment