views
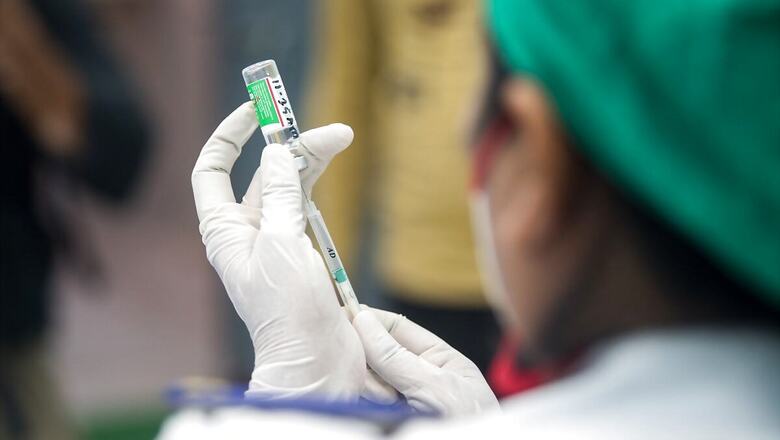
The Central government on Thursday said that it was working together with Pfizer for the earliest possible import of its Covid-19 vaccines and that compulsory licensing was not a very attractive option.
In a detailed statement issued on Thursday, the government also said that no country in the world was giving vaccines to children and there was no WHO recommendation on vaccinating children. The statement said that India has waived off the trial requirement altogether for the well-established vaccines manufactured in other countries, but vaccines are in short supply in the world and was not easy to procure them at short notice through steps like global tenders by the states.
However, as opposed to India?s claimes, countries like the US and the UAE are already vaccinating children as young as 12.
“As soon as Pfizer indicated vaccine availability, Central Government and the company are working together for the earliest possible import of the vaccine. We reiterate our request to all international vaccine makers to come and make in India – for India and for the world,” NITI Aayog Member VK Paul said in the statement issued Thursday. Under attack from the Opposition for the vaccine shortage in the country, the Centre made a case that the Indian government ran the entire vaccine program from January to April in a “well-administrated” way compared to the situation in May when it had to come out with a liberalised vaccine policy “as a result of the incessant requests being made by the states to give states more power.”
It has pointed out that the fact that global tenders have not given any results only reaffirm what the Centre has been telling the states from day one – “that vaccines are in short supply in the world and it is not easy to procure them at short notice.” Vaccination speed in the country has almost halved since it was opened for the 18-44 group from May 1, compared to April.
Also read: Our Vaccine Highly Effective on New Covid Variant, Suitable for 12 Yrs and Above: Pfizer to Govt
The statement points to several myths on vaccination “due to distorted statements, half-truths and blatant lies” in an apparent reference to the incessant attack from the Congress. Former prime minister Manmohan Singh last month asked Prime Minister Narendra Modi to invoke compulsory licensing to increase vaccine availability. “Compulsory Licensing is not a very attractive option since it is not a ‘formula’ that matters, but active partnership, training of human resources, sourcing of raw materials and highest levels of bio-safety labs which is required. Tech transfer is the key and that remains in the hands of the company that has carried out R&D. Infact, we (Centre) have gone one step ahead of Compulsory Licensing and are ensuring active partnership between Bharat Biotech and 3 other entities to enhance production of Covaxin. Similar mechanism is being followed for Sputnik. Moderna had said in October 2020 that it will not sue any company which makes its vaccines, but still not one company has done it, which shows licensing is the least of the issues. If vaccine-making was so easy, why would even the developed world be so short of vaccine doses?” Paul said in his statement.
He claimed that the Centre had done a lot to buy vaccines from abroad and had remained engaged continuously with all the major international vaccine manufacturers right from mid-2020, including multiple rounds of discussions have happened with Pfizer, J&J & Moderna. The statement read, “Government offered all assistance to have them supply and/or manufacture their vaccines in India. However, it is not that their vaccines are available in free supply. We need to understand that buying vaccines internationally is not similar to buying ‘off the shelf’ items. Vaccines are in limited supply globally, and companies have their own priorities, game-plans and compulsions in allocating finite stocks. They also give preference to countries of their origin just as our own vaccine makers have done unhesitatingly for us,” Paul has said. He said Russia has already sent two tranches of Sputnik V vaccines and Indian companies that would start manufacturing the vaccine very soon as well. “The Central Government has proactively eased entry of vaccines approved by US FDA, EMA, UK?s MHRA and Japan?s PMDA, and WHO?s Emergency Use Listing into India in April. These vaccines will not need to undergo prior bridging trials. The provision has now been further amended to waive off the trial requirement altogether for the well-established vaccines manufactured in other countries. No application of any foreign manufacturer for approval is pending with the drugs controller.?
Also read: From Vaccines To Quad: Why Jaishankar?s US Visit Can Be Shot In Arm For Ties With US
Paul said India is enabling more domestic companies to produce vaccines since early 2020. “3 other companies/plants will start production of Covaxin apart from enhancing Bharat Biotech’s own plants, which have increased from 1 to 4. Covaxin production by Bharat Biotech is being increased from under 1 Cr per month to 10 Cr month by October. Additionally, the three PSUs will together aim to produce upto 4.0 Cr doses by December. Serum Institute is ramping up Covishield production of 6.5 crore doses per month to 11.0 crore doses per month. GoI is also ensuring in partnership with Russia that Sputnik will be manufactured by 6 companies coordinated by Dr Reddy’s,” Paul said.
He said efforts by Zydus Cadila, BioE as well Gennova for their respective indigenous vaccines were also being supported through liberal funding under the Covid Suraksha scheme and the development of Bharat Biotech’s single-dose intranasal vaccine “could be a game-changer for the world.” India has estimated production of over 200 crore doses by India’s vaccine industry by the end of 2021. “How many countries can even dream of such an enormous capacity, and that too across conventional as well as cutting-edge DNA and mRNA platforms? GoI and vaccine manufacturers have worked as one Team India in this mission with seamless engagement on daily basis,” Paul said in his statement.
Regarding the allegation that the Centre had abdicated its responsibility to the states for the 18-44 group, Paul said the Centre has merely enabled states to try procuring vaccines on their own, on their explicit requests. “The states very well knew the production capacity in the country and what the difficulties are in procuring vaccines directly from abroad. But states, who had not even achieved good coverage of healthcare workers and frontline workers in 3 months wanted to open up the process of vaccination and wanted more decentralisation. Health is a state subject and the liberalised vaccine policy was a result of the incessant requests being made by the states to give states more power,” Paul said. He said states are being informed in advance of vaccine availability, which is going to increase in near future. “In the non-GoI channel, states are getting 25% of the doses and private hospitals are getting 25% doses. However the hiccups and issues faced by the people in the administration of these 25% doses by states leave a lot to be desired. The behaviour of some of our leaders, who in spite of full knowledge of the facts on vaccine supply, appear on TV daily and create panic among the people is very unfortunate. This is not the time to play politics,? Paul said.
On vaccinating children, Paul said trials in children in India are going to begin soon and vaccinating them has to be a decision taken by scientists after adequate data is available based on trials. “Vaccinating children should not be decided on the basis of panic in Whatsapp groups and because some politicians want to play politics. As of now, no country in the world is giving vaccines to children. Also, WHO has no recommendation on vaccinating children. There have been studies about safety of vaccines in children, which have been encouraging,” Paul said.
Read all the Latest News, Breaking News and Coronavirus News here. Follow us on Facebook, Twitter and Telegram.
















Comments
0 comment