views
X
Research source
Often, amounts of compounds are given in grams and need to be converted to moles. This conversion can help give you a clearer picture of the number of molecules you're working with rather than dealing with weight, which can change between molecules. Although the conversion is simple, there are a number of important steps that need to be followed. Using this method, you can learn how to convert grams into moles.
Calculating the Molecular Mass
Gather the necessary tools for solving a chemistry problem. Having everything you need easily accessible will simplify the process of solving the assigned problem. You will need the following: A pencil and paper. Calculations are easier to solve when you write them out. Be sure to show all your steps to get full credit. A periodic table. You will need to be able to find atomic weight of elements using the periodic table. A calculator. Calculators are necessary to simplify calculations of complex numbers.
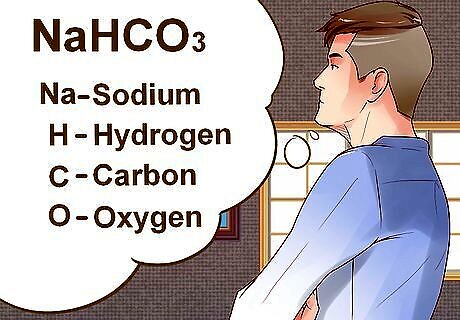
Identify the elements in the compound that you need to convert into moles. The first step in calculating molecular mass is identifying each element that composes the compound. It is easy to distinguish elements because abbreviations contain only one or two letters. If a compound is abbreviated with two letters, the first will be capitalized while the second will be lowercase. For example, Mg is the abbreviation for magnesium. The compound NaHCO3 has four elements in it: sodium (Na), hydrogen (H), carbon (C), and oxygen (O).
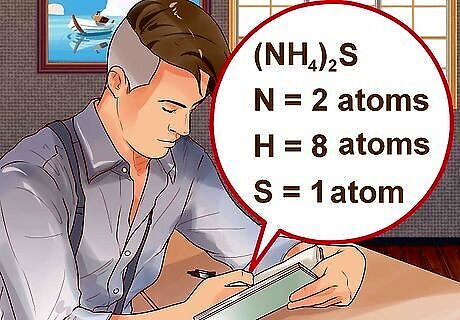
Determine the number of atoms that each element contributes to the compound. You must know how many atoms of each element are present to calculate the molecular mass. The number of atoms each element contributes will be written in a subscript next to the element. For example, H2O has two atoms of hydrogen and one atom of oxygen. If a compound has parentheses followed by a subscript, each element within the parentheses gets multiplied by the number in the subscript. For example, (NH4)2S has two atoms of N, eight atoms of H, and one atom of S.
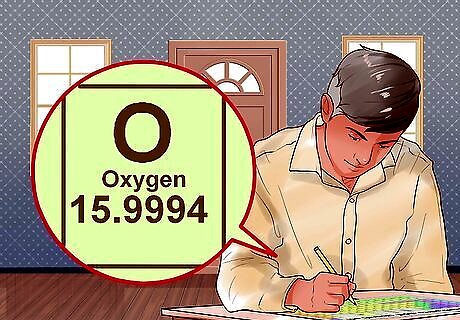
Write down the atomic weight of each element. A periodic table is the easiest way to find the atomic weight of an element. Once you locate the element on the table, the atomic weight is usually found underneath the symbol for that element. The atomic weight, or mass, or an element is given in atomic mass units (amu). For example, the molecular weight of oxygen is 15.99.
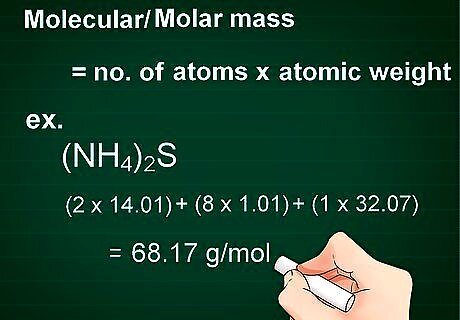
Calculate molecular mass. The molecular mass of a substance is calculated as the number of atoms of each element multiplied by the atomic weight of that element. Knowing the molecular mass is necessary to convert grams to moles. Multiply the number of atoms each element contributes to the compound by the atomic weight of that element. Add the total weight of each element in the compound together. For example, (NH4)2S has a molecular weight of (2 x 14.01) + (8 x 1.01) + (1 x 32.07) = 68.17 g/mol. Molecular mass is also referred to as molar mass.
Converting Grams to Moles
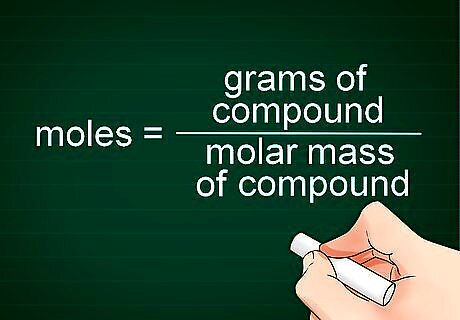
Set up the conversion formula. The number of moles you have of a compound can be calculated by dividing the number of grams of the compound by the molecular mass of the compound. The formula looks like this: moles = grams of compound/molar mass of compound
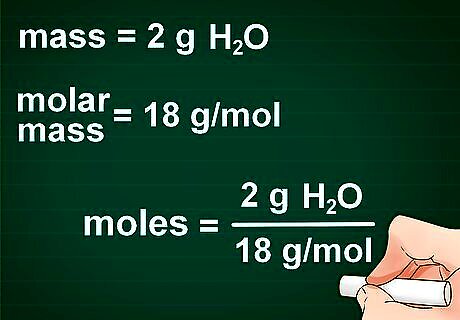
Plug your numbers into the formula. Once you have properly set up the formula, the next step is just putting your calculations into the correct part of the formula. An easy way to check that you have everything in the right place is by the units. Canceling out all the units should leave you with just moles.
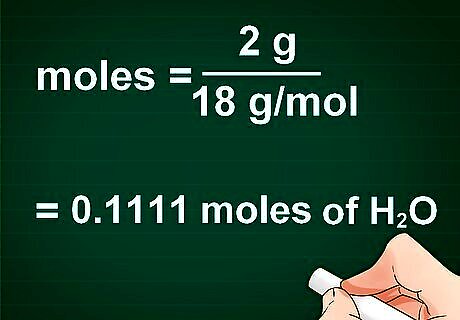
Solve the equation. Using a calculator, divide the number of grams by the molar mass. The result is the number of moles in your element or compound. For example, imagine you have 2 g of (NH4)2S and you want to convert it to moles. The molecular mass of (NH4)2S is 68.17g/mol. Divide 2 by 68.17, and you have 0.0293 moles of (NH4)2S.
















Comments
0 comment