
views
Understanding Viscosity

Define viscosity. Viscosity measures a liquid’s resistance to flow. A fluid with high viscosity flows very slowly, like honey. A fluid with low viscosity flows quickly, like water. The unit for viscosity is a pascal second (Pa s).

Define the equation for viscosity. This experiment will take measurements of a sphere and its passage through liquid to calculate viscosity. The equation for viscosity is [2(ps-pl)ga]/9v where ps is the density of the sphere, pl is the density of the liquid, g is acceleration due to gravity, a is the radius of the sphere, and v is the velocity of the sphere.

Understand the variables in the viscosity equation. Density is mass per unit volume of an object and is designated with a p. In this equation, you need to measure the density of both the sphere, ps, and the liquid, pl, it is passing through. The radius of the sphere, a, can be found by measuring the circumference of the sphere and dividing that by 2π. The acceleration due to gravity, g, is a constant dependent on the atmosphere of the planet you’re on. In this case, you are on earth so g is 9.8m/s. The velocity of the sphere, v, is calculated during the experiment and is the time it takes an object to travel a specific distance in meters per second (m/s).
Measuring Viscosity
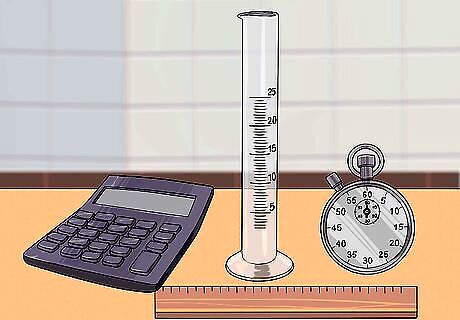
Gather the necessary materials for the experiment. To calculate viscosity of a liquid, you will need a sphere, a graduated cylinder, a ruler, a stopwatch, the liquid in question, a scale, and a calculator. This experiment has many steps, but when followed correctly, they will allow you to calculate the viscosity of any liquid. The sphere can be a small marble or steel ball. Make sure its diameter is no greater than half the diameter of the graduate cylinder so it can easily be dropped into the cylinder. A graduated cylinder is a plastic container that has graded markings on the side that allow you to measure volume. You can use a watch instead of a stopwatch, but your measurements will be more accurate with a stopwatch. The liquid must be clear enough to see the marble as it’s dropped through the liquid. Try testing many different liquids with different flow rates to see how their viscosities differ. Some common liquids you could try including water, honey, corn syrup, cooking oil, and milk.
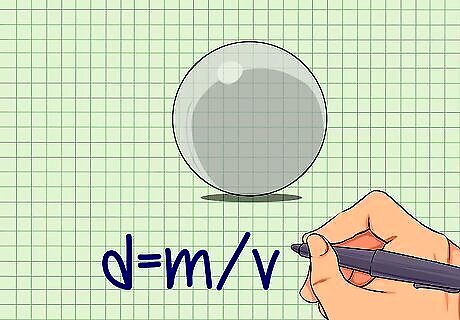
Calculate the density of your chosen sphere. The density of both the sphere and the liquid are needed to perform the viscosity calculation. The formula for density is d = m / v {\displaystyle d=m/v} d=m/v, where d is density, m is the mass of the object, and v is the volume of the object. Measure the mass by placing the sphere on a balance. Record the mass in grams (g). Determine the volume of a sphere using the formula V= (4/3) x π x r, where V is volume, π is the constant 3.14, and r is the radius of the sphere. You can find the radius by measuring around the center of the sphere to get its circumference and then dividing the circumference by 2π. You can also find volume by measuring the displacement of water in a graduated cylinder. Record the initial water level, place the sphere in the water, and record the new water level. Subtract the initial from the new water level. This number equals the volume of your sphere in milliliters (mL). Calculate density with the formula d = m / v {\displaystyle d=m/v} d=m/v. The unit for density is g/mL.
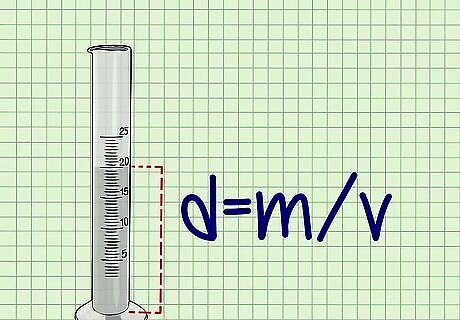
Determine the density of the liquid you are measuring. Using the same density formula from above, you will next calculate the density of the liquid in question. Measure the mass of the liquid by first weighing the empty graduated cylinder. Pour your liquid into the graduated cylinder and then weigh it again. Subtract the mass of the empty cylinder from that of the cylinder with the liquid in it to obtain the mass of the liquid in grams (g). To find the volume of the liquid, simply determine the amount of liquid you poured into the graduated cylinder by using the graded markings on the side of the cylinder. Record the volume in milliliters (mL). Use the formula d = m / v {\displaystyle d=m/v} d=m/v and your measurements to calculate the density of the liquid in g/mL.
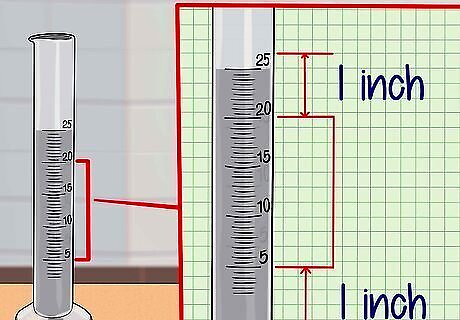
Fill and mark the graduated cylinder. First, fill your graduated cylinder with the liquid to be measured. Then, mark the positions at the top and bottom of the cylinder. Slowly pour your experimental liquid into the graduated cylinder, filling the cylinder about halfway to three-quarters of the way to the top. Draw a mark at the top of the cylinder about 2.5 centimeter (1 in) (1 in) from the top of the liquid. Draw a second mark about 2.5 centimeter (1 in) (1 in) from the bottom of the graduated cylinder. Measure the distance between the top and bottom marks. Place the bottom of the ruler at the bottom mark and record the distance to the top mark.
Record the time it takes for the ball to drop between the marks. Drop the ball into the liquid and start the stopwatch when the bottom of the ball reaches the mark at the top of the cylinder. When the ball reaches the mark you made at the bottom of the cylinder, stop the stopwatch. Liquids with low viscosities are going to be more difficult to measure with this method because it will be harder to accurately start and stop the stopwatch. Repeat this step at least three times (the more times you repeat, the more accurate your measurement will be) and average the three times together. To find the average, add up the times for each trial and divide by the number of trials you performed. This works best if the ball is small enough that the flow around the ball is truly viscous and far from turbulent. The ball must also be much smaller than the container so the ball can be dropped at least 10 ball-radii from the side walls.
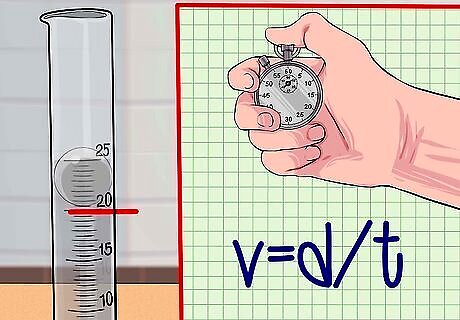
Calculate the velocity of the sphere. Velocity is a measurement of distance traveled over elapsed time to travel that distance. The formula for velocity is v = d / t {\displaystyle v=d/t} v=d/t where v is velocity, d is distance traveled, and t is time. Using your measurements, plug them into the equation v = d / t {\displaystyle v=d/t} v=d/t to find the velocity of the sphere.
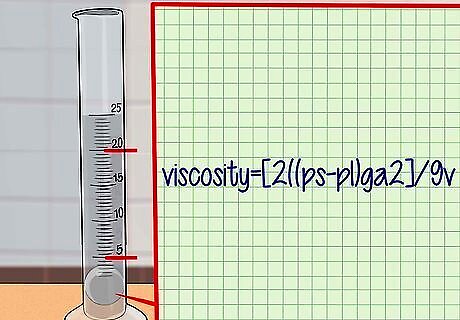
Calculate the viscosity of the liquid. Plug the information you have obtained into the formula for viscosity: viscosity = [2(ps-pl)ga]/9v where ps is the density of the sphere, pl is the density of the liquid, g is acceleration due to gravity (a fixed value of 9.8 m/s), a is the radius of the sphere, and v is the velocity of the sphere. For example, let’s say the density of your fluid is 1.4 g/mL, the density of your sphere is 5 g/mL, the radius of the sphere is 0.002 m, and the velocity of the sphere is 0.05 m/s. Plugging into the equation: viscosity = [2(5 – 1.4)(9.8)(0.002)^2]/(9 x 0.05) = 0.00062784 Pa s

















Comments
0 comment