views
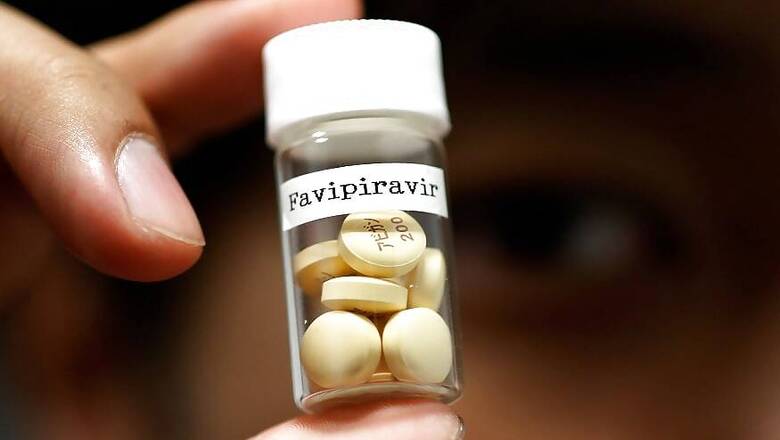
New Delhi: Mumbai-based Glenmark Pharmaceuticals has received approvals from Drug Controller General of India to manufacture and market Favipiravir, an anti-viral drug, to treat Covid-19 patients. The drug is likely to be available in the market in the next few days. What is this drug and what improvement has it shown in Covid-19 patients? News18 explains:
What is Favipiravir?
Favipiravir is an anti-viral drug and it is approved in Japan for treating influenza. It is currently being tested in 18 clinical trials for Covid-19 and results from two studies have shown a positive outcome, while data from other trials is awaited.
What is the Glenmark drug approved by the Drug Controller General of India?
Based on Phase-3 data, the company obtained approval for manufacture and marketing of antiviral drug Favipiravir, which has been branded as FabiFlu, an oral medication. The approval is for emergency restricted use only for treatment of mild to moderate Covid-19 patients. The approval’s restricted use entails responsible medication use where every patient must have signed informed consent before treatment initiation.
According to a press release issued by Glenmark on Saturday, June 20, the company successfully developed the active pharmaceutical ingredient and formulation for FabiFlu through its own in-house R&D team.
“Glenmark filed the product for clinical trial with India’s drug regulator DCGI and became the first pharmaceutical company in India to receive approval for conducting phase 3 clinical trial on mild to moderate COVID-19 patients," the press release said.
What improvement has it shown in Covid-19 patients and what studies has Glenmark cited?
Glenmark claimed that Favipiravir shows clinical improvements of up to 88 per cent in Covid-19, with rapid reduction in viral load by four days. The drug will be available as a prescription-based medication for Rs 103/tablet, with recommended dose being 1800 mg twice daily on day 1, followed by 800 mg twice daily up to day 14.
In India, a randomised multi-centre study was done on Indian patients to test the drug’s efficacy and safety with standard of care vs standard of care alone in mild to moderate Covid-19, the company said. The study enrolled 150 patients. The study’s details are yet to be published in a peer-reviewed paper.
In its press release, the company cited four studies; two from China and one reach from Russia and Japan. One of the studies done on 80 patients in China studied the efficacy of Favipiravir vis-à-vis anti-retroviral drugs Lopinavir and Ritonavir. Favipiravir treatment led to a quicker reduction in viral load compared to the other drugs. In the second Chinese study, 236 patients were enrolled.
Favipiravir showed a better rate of clinical recovery on seventh day compared Umifenovir, another antiviral drug. It also led to a quicker relief from fever and cough. The observational study in Japan comprising 2,141 patients with mild to moderate symptoms showed clinical improvement using Favipiravir.
Is the government conducting trials to test the efficacy of this drug?
Yes. The Council of Scientific and Industrial Research (CSIR) had done end-to-end synthesis of Favipiravir in April. Earlier this week, CSIR got approvals from DGCI to conduct multi-centre Phase-II trials of the drug.
















Comments
0 comment