views
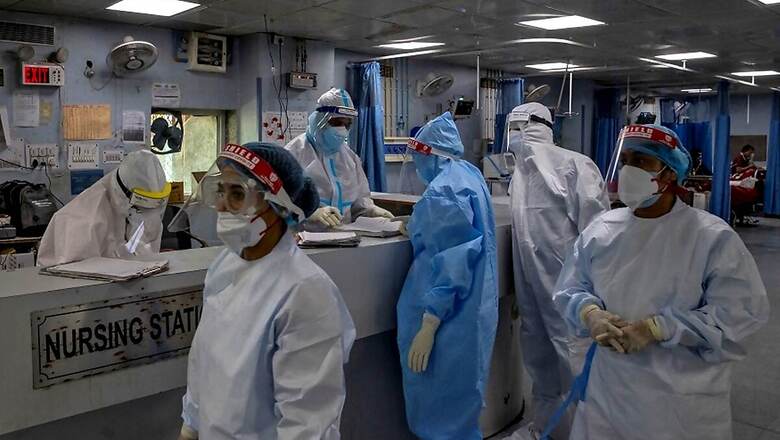
The ICMR on Thursday sought participation of institutions and hospitals identified as dedicated coronavirus hospitals or health centres as part of its project to establish 'National Clinical Registry of COVID-19' to help improve treatment outcomes and analyse trends in the progression of the pandemic.
The aim is to develop a registry to collect data regarding clinical and laboratory features, treatments, and outcomes of hospitalized COVID-19 patients in India.
The objective is also to study the frequency, clinical and laboratory features, treatments, and outcomes of COVID-19 related multisystem inflammatory disorder in children and adolescents by analyzing the national COVID-19 registry, the ICMR said. Data would be collected from 100 hospitals across the country as part of the project.
"The NCRC will aim at collecting good quality, real-time clinical data to inform evidence-based clinical practice, research, formulating guidelines and policy making," the apex health research body.
"ICMR invites a letter of intent from institutions/ hospitals identified as dedicated COVID Hospitals or dedicated COVID Health Centres under the project to establish National Clinical Registry of COVID-19," the letter of invitation said.
The duration of the study will be one year. Any COVID-19 lab confirmed and hospitalized patient of any age and meeting the inclusion criteria for the study will be enrolled, the document stated.
Data entry of clinical and laboratory parameters as well as patient management and outcomes will be filled in a predesigned structured proforma on an electronic platform by participating sites across the country. "The data will be used to generate hypotheses for various parameters of the COVID-19 disease and the registry platform will be utilized in the future for interventional studies," the document said.
Dedicated COVID -19 hospitals and health centres will serve as primary sites for data collection. These sites will be trained, mentored and supervised by 15 medical institutes of repute across the country. The initiative comes in view of a pressing need for collection of systematic data on clinical signs and symptoms, laboratory investigations, management protocols, clinical course of COVID-19 disease, disease spectrum and outcomes of patients.
"Such data will serve as an invaluable tool for formulating appropriate patient management strategies, predicting disease severity, patient outcomes etc," the letter of intent said.
In view of this, Ministry of Health, Indian Council of Medical Research (ICMR), New Delhi and All India Institute of Medical Sciences (AIIMS) New Delhi have proposed to launch a National Clinical Registry for COVID-19 (NCRC).
The data collected will be stored in-central server/ NIC cloud and analysis will be done centrally by a team of scientists at ICMR. The data entered by individual sites will be accessible to them on the central server. Analyzed data will be shared periodically with all sites. The expected output of developing and maintaining a NCRC include generating weekly reports – epidemiological and clinical reports – from the registry data which will be published on ICMR/MOHFW website, formulating patient management protocols and develop policy guidelines for decision making.
It is expected to help understand the predictors of disease severity and optimize patient management protocols accordingly and to understand variations (if any) in clinical signs and symptoms and spectrum of disease in India as compared to other affected countries, publish data periodically in peer reviewed journals and to make evidence-based decisions on implementing various interventions in COVID-19 patients.
According to the document, all the Institutions involved in the study will be funded by the Indian Council of Medical Research (ICMR). A uniform budget will be prepared for the sites (COVID-19 hospitals) and the mentor institutes.
Fifteen institutes include Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh which would train and mentor sites from Jammu and Kashmir, Ladakh, Punjab, Haryana, Chandigarh, Uttarakhand, AIIMS in Delhi from Delhi and Bihar, AIIMS in Jodhpur would supervise sites in Rajasthan, King George Medical University, Lucknow from Uttar Pradesh Jawaharlal Institute of Postgraduate of Medical Education and Research in Puducherry fromTamil Nadu, Andhra Pradesh, Puducherry and North-Eastern Indira Gandhi Regional Institute of Health and Medical Sciences, Shillong would supervise sites from Assam, Meghalaya, Sikkim, Tripura, Manipur, Mizoram, Arunachal Pradesh and Nagaland.

















Comments
0 comment