views
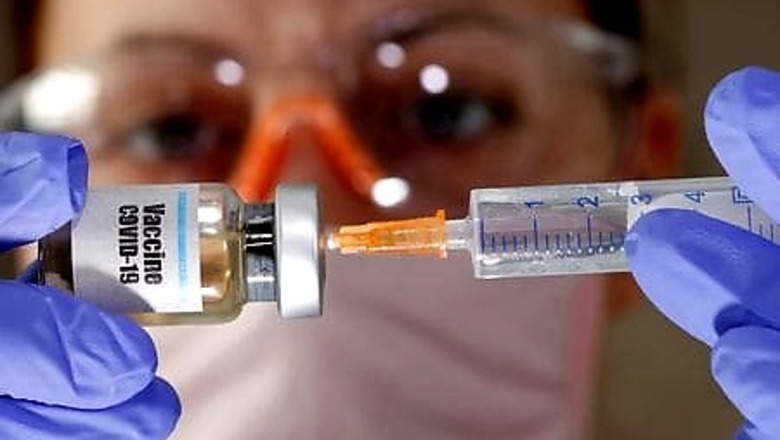
BRUSSELS: It is hard enough developing a vaccine in record time to halt a global pandemic. But what if you need to print the instructions with every dose in Portuguese, Lithuanian and Greek?
Drugmakers are asking the European Union to loosen rules that require medicines sold in the bloc to include full documentation in 24 separate languages, worried that this could slow down the rapid deployment of hundreds of millions of doses.
“We need an early agreement from EU authorities on the language to be used on the packs and labels for COVID-19 vaccines,” said Michel Stoffel, head of regulatory affairs at Vaccines Europe, which represents big vaccine makers including GlaxoSmithKline, Sanofi and AstraZeneca.
He told Reuters the industry was pushing EU regulators to quickly choose one language for all 27 EU states for labelling, packaging and instructions on possible COVID-19 vaccines.
The EU’s executive Commission promised in June it would temporarily soften language requirements for COVID-19 vaccines, but has not yet put forward a proposal. A spokesman said work was underway to be flexible without compromising on safety.
An EU official said Brussels was considering having printed information in a limited set of languages. Other versions would be available online.
An industry official said even that might be too difficult: labels may not have enough space for more than two versions.
Consumer groups say leaving any languages off of packaging could hurt patients, particularly those less capable of looking up details online.
“The urgency of getting a vaccine should not be an excuse for companies to cut corners on consumer protection,” said Monique Goyens, the head of BEUC which represents major European consumer organisations.
The EU translates all its rules into all member languages. Commission staff use English, French and German as working languages.
Disclaimer: This post has been auto-published from an agency feed without any modifications to the text and has not been reviewed by an editor



















Comments
0 comment