views
Observing the Properties of the Liquid
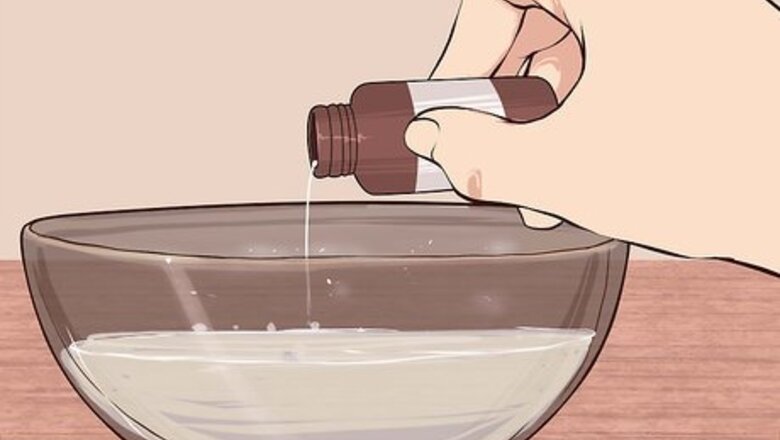
Mix a sample of the liquid with a known acid. Acids and bases are well known for neutralizing each other on contact. This neutralization reaction can be recognized because it releases heat and gas, which causes the two reactants to fizz. Choose a well known, safe acid to do this reaction. Put a sample of your liquid into a bowl or dish. Add your acid with a dropper. If you see fizz forming in the bowl, your liquid is most likely a base. Choose a dilute acid such as vinegar. Do not use strong acids like hydrochloric acid or nitric acid.
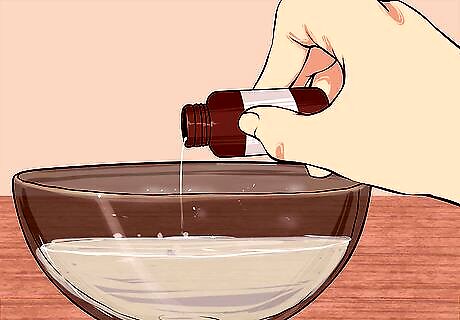
Mix a sample of the liquid with a known base. Add the base to a bowl and then add your liquid to the base with a dropper. If you see the fizzing and bubbling of a neutralization reaction, your liquid is most likely an acid. There is no need to use a strong base. Something like baking soda will work well.
Determine whether the liquid is safe to drink. You can distinguish acids and bases by taste, but this should never be done with “mystery liquids.” If you know what liquid you are testing, then you can decide if it is safe to drink. Only drink liquids that are intended for human consumption, such as water, milk, orange juice.
Taste the liquid if it is safe to do so. If you determine that your liquid is safe to drink, taste a sample. You can distinguish acids by their sour taste (lemons are high in citric acid). Bases can be identified by a bitter taste (mustard is basic).
Using Colored Indicators

Choose an indicator. An indicator is a chemical (usually a type of dye) that will change colors predictably when exposed to an acid or base. Litmus will turn from blue to red in the presence of acid and from red to blue in the presence of a base. Red cabbage juice contains chemicals known as anthocyanins that turn red when exposed to acid, blue when exposed to bases, and yellow when exposed to a strong base. Your indicator can be in a liquid form, or you can use strips of paper coated with the indicator, known as pH strips.

Expose the indicator to a sample of liquid. If you choose to use pH strips, simply dip the strip into the liquid. Allow the strip to air dry momentarily. If you choose to use a liquid indicator, put the indicator in a clear container (so that you do not distort the color). Add the sample liquid to the indicator by dropping it in with a dropper.
Observe the color change. Whether you use the pH strips or the liquid indicator, the color change should be the same. Some indicators, such as litmus, will only tell you if your solution is an acid or a base. Other indicators, such as red cabbage juice, can be compared to a universal indicator that will give you an approximate pH range of your liquid.
Measuring the pH
Choose a pH meter. There are several different methods of measuring pH. The hydrogen electrode method is considered the most accurate standard, but is inconvenient for most applications. The most commonly used method is the glass diode method. It is easy to use and you get the same results (or close) each time you use it. If you are using very small sample sizes, you might consider a semi-conductor type pH meter. You can find pH meters at some gardening stores and places that sell laboratory equipment in addition to big-box stores and online.
Prepare your liquid. You want to make sure that your sample is as close to uniform as possible. Shake or stir the sample before measuring with the pH meter. Be sure that there are no solid particles in your sample, and that the sample is at a stable temperature. This way you will get the most accurate reading.
Prepare the meter. Each meter will differ slightly, and you should follow the manual given with the meter you choose. Usually, preparation will consist of putting 1 electrode in the sample and another electrode in a solution of known pH. Be sure that both electrodes are clean.

Measure the pH of your liquid. Submerge both electrodes into the proper liquids. The difference in voltage between the 2 electrodes is related to the pH of the 2 solutions. This allows the meter to calculate the pH of your solution.




















Comments
0 comment