views
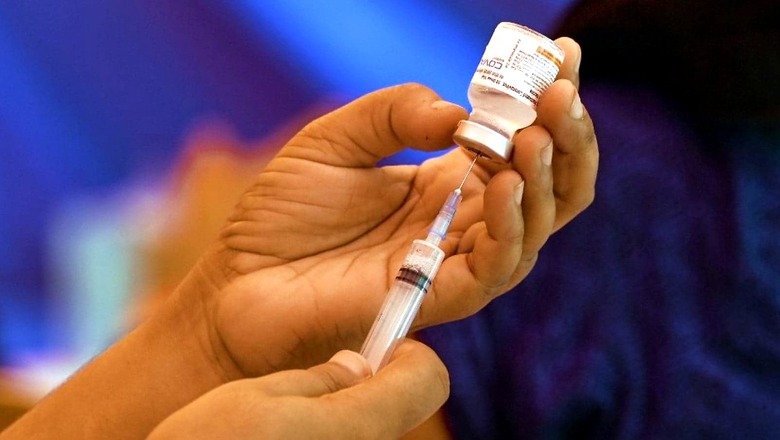
Ocugen Inc, Bharat Biotech’s partner for USA and Canada for COVID-19 vaccine Covaxin on Friday said it has submitted a request to the US Food and Drug Administration (FDA) for Emergency Use Authorization (EUA) of the jab for paediatric use.
The submission is based on results of a Phase 2/3 paediatric clinical trial conducted by Bharat Biotech in India with 526 children 2-18 years of age, which bridged immunogenicity data to a large, Phase 3 safety and efficacy clinical trial in nearly 25,800 adults in India, Ocugen said in a regulatory filing.
“Filing for Emergency Use Authorization in the US for paediatric use is a significant step toward our hope to make our vaccine candidate available here and help combat the COVID-19 pandemic, Shankar Musunuri, Chairman of the Board, Chief Executive Officer and Co-Founder of Ocugen said.
Some research suggests that people are seeking more choices when selecting a vaccine, especially for their children. Having a new type of vaccine available will enable people to discuss with their child’s physician the best approach for them to lower their child’s risk of contracting COVID-19, he further said. “The inactivated virus platform has been used for decades in vaccines for the paediatric population and, if authorized, we hope to offer another vaccine option to protect children as young as two years,” he added.
A Phase 2/3, open-label, multi-center study was conducted in India from May 2021 to July 2021 to evaluate the safety, reactogenicity and immunogenicity of the whole-virion inactivated Vaccine in healthy volunteers in the 2-18 age group. Covaxin was evaluated in three age groups: 2-6 years, 6-12 years and 12-18 years. All participants received two doses of the vaccine 28 days apart, it said.
Covaxin was recently awarded Emergency Use Listing by the World Health Organization.
Read all the Latest News here

















Comments
0 comment