views
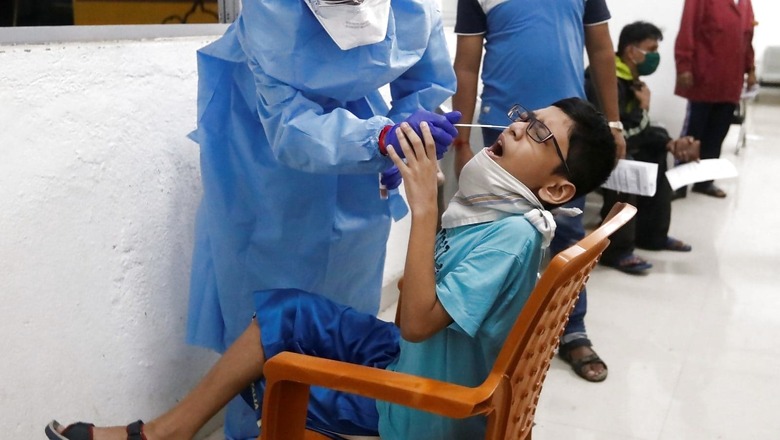
The Covid-19 vaccination drive for children is likely to begin by the second half of November, News18.com has learnt. The subject expert committee (SEC) under the country’s drug regulator approved the use of Bharat Biotech’s Covaxin in children between 2 and 18 years of age on Tuesday. The drive will first cater to children suffering from chronic or severe health conditions; the priority list of diseases may take up to three weeks to get ready.
The recommendation is being reviewed by the Drugs Controller General of India (DCGI), and once approved, the process of rollout will begin next month.
“Once the DCGI approves the vaccine, the members of National Technical Advisory Group on Immunisation (NTAGI) will take over and read the interim data submitted by the company for approval. They may seek additional inputs from Bharat Biotech,” said a senior government official requesting anonymity.
“Based on the efficacy and safety data, NTAGI will complete the priority list for vaccination among children. The finalisation of the list could take up to three weeks. This list will be the backbone of the drive and will help us create concrete plans.”
According to a source, who is also a member of NTAGI, it needs to be understood how many doses Bharat Biotech can provide for the immediate rollout, and for the next three months. “After making the right balance for allocation of Covaxin for adults and children, we are likely to start inoculation among kids by the middle or second half of November.” He said his concern was that the pace of vaccination for adults should nowhere be “compromised” in the children vaccination process.
Covaxin is the second Covid-19 vaccine to be approved for emergency use among children in India after Zydus Healthcare’s ZyCoV-D for children above the age of 12. However, Covaxin is one of the first vaccines to be approved worldwide for children of the 2-18 age group.
Company to submit data after every 15 days
The two-dose Covaxin will be administered to children with the gap of 28 days between first and second dose. The vaccine dosage for children would be 0.5ml, the same as for adults.
The SEC panel has recommended the company to continue the study according to the approved protocols. The panel has also asked the company to submit the safety data, which also includes the data on adverse events, or side-effects every fortnight for the first two months and monthly thereafter.
The similar riders were being put out when Covaxin was first approved by the regulator for the use among adults.
Bharat Biotech said on Tuesday it has submitted data from clinical trials in the 2-18 years’ age group for Covaxin to Central Drugs Standard Control Organisation (CDSCO). “This represents one of the first approvals worldwide for Covid-19 vaccines for the 2-18 age group. We now await further regulatory approvals from the CDSCO prior to product launch and market availability of COVAXIN for children.”
Read all the Latest News , Breaking News and IPL 2022 Live Updates here.

















Comments
0 comment