views
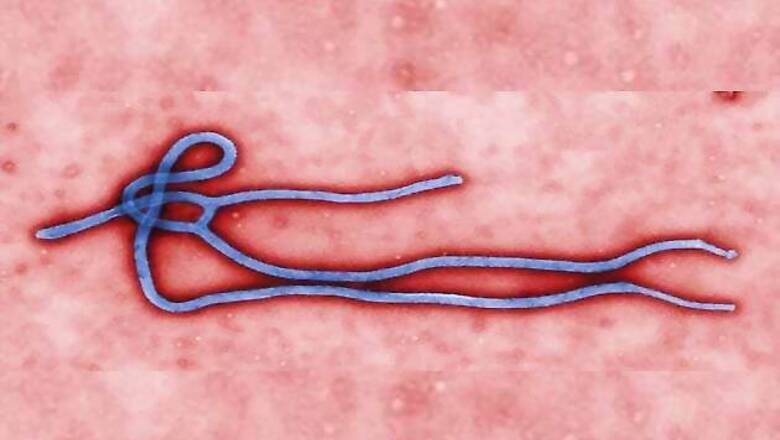
Geneva: Drug and vaccine companies are racing to conduct clinical trials of potential treatments for Ebola but it will be 2015 before there are any initial results and much later before significant quantities could be available, executives said on Friday.
In interviews on the sidelines of a meeting hosted by the World Health Organization (WHO), they said that efforts would focus on developing safe and efficient products for human use that could win fast-tracked regulatory approval.
ZMapp, by Mapp Biopharmaceutical Inc., has been given to seven infected people, including two American aid workers and a Briton who all recovered, but it remains unproven and supplies have run out. The US government pledged up to $42.3 million this week to accelerate its testing.
Dr. Larry Zeitlin, president of the California-based Mapp Biopharmaceutical, said that Washington's support was vital to conduct early stage safety studies of the experimental drug as the jury is still out on both its safety and efficacy.
"The US support will enable us to figure out what the appropriate dose is and scale up manufacturing. With a drug you have not only to make it, but make it consistently to the same quality. The award given us is for 18 months. We will probably be in human trials beginning in 2015," Zeitlin told Reuters.
"We don't have data indicating whether ZMapp is safe in humans, we don't have data that it works in humans. That is the whole point of performing clinical trials," he said.
At this point, Zeitlin said that he expected most of the production to go into clinical trials rather than so-called "compassionate care".
ZMapp is among eight experimental drugs and two candidate vaccines deemed by the WHO to have potential against the virus that has killed at least 1,900 people in West Africa since March. The WHO has warned that 20,000 people could be at risk.
The current strain of Ebola has an overall death rate of about 50 per cent.
On Thursday, the UN agency called for pharmaceutical companies and regulatory agencies to work together to accelerate development of the most promising treatments.
The two-day talks, attended by nearly 200 experts, are due to end later on Friday with a WHO statement.
"RAMPING UP"
Drugs include AVI 7537, made by Sarepta Therapeutics Inc., which was tested on animals and completed phase 1 human safety studies, but had to be put to the side in late 2011 due to US budget cuts, said Dr. Michael Wong, senior medical director for infectious diseases at Sarepta.
"We still have drug substance that is still stable. We are ramping up another human trial," Wong told Reuters.
From 60 to 80 per cent of rhesus monkeys given AVI 7537 survived, while all of those in the test group died, he said.
A phase 1 human safety study under the US Food and Drug Administration found "no safety or tolerance issues at all".
"We are looking at ways we can support the WHO if they feel the best way of looking at some agents is through some form of a trial," Wong said.
"Because the epidemic is unprecedented and still rolling, they are looking at several different approaches. The theme is to try to do a thorough, careful and ethical job but to do it fast."
Human safety trials are due to begin this week on a vaccine from GlaxoSmithKline Plc and later this year on one from NewLink Genetics Corp
"We are working on a vaccine and have been asked by WHO to make it available as quickly as possible to help control this outbreak. Phase 1 studies started this week at NIH (the U.S. National Institutes of Health)," Dr. Ripley Ballou, of GlaxoSmithKline Biologicals SA, Rixensart, Belgium told Reuters.
"We hope to have at the end of the year a good sense if the vaccine is safe and well-tolerated in five trials, involving 120-150 people. We'll have the data that we need by the end of the year but actually the studies will go for one year.
"Most important is that we can select the dose for the next phase," Ballou said.
The WHO talks, marked by testimony from health officials from Guinea, Liberia and Nigeria, were "eye-opening," he said.
"For anybody who is contemplating product development it reinforces how challenging this is going to be, it is a real complex undertaking."

















Comments
0 comment