views
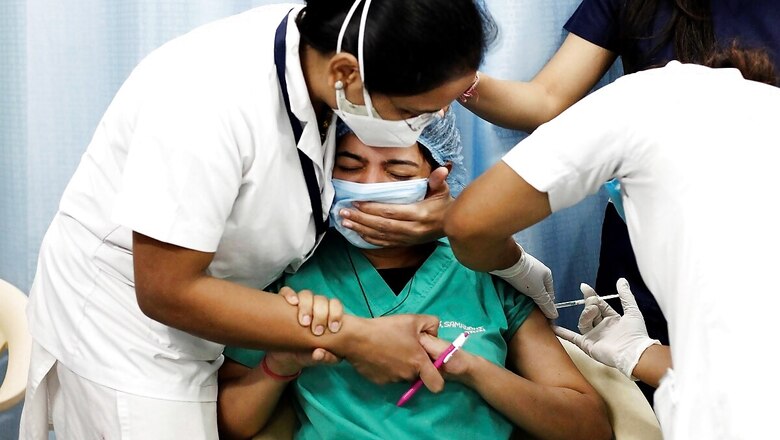
Amid reports of possible side-effects of Oxford-AstraZeneca’s COVID-19 vaccine and its suspension in some European countries, the government reiterated on Wednesday said there was “no signal of concern” regarding its use in the country as of now.
“The scientific community that studies Adverse event following immunization (AEFI) has given NO SIGNAL for Covishield causing blood clotting, Dr VK Paul, NITI Aayog member (Health) told a press conference in Delhi as India battles a fresh wave of infections.
After concerns about AstraZeneca’s vaccine relationship with thrombotic events in people who received the vaccine came to light, about 10 European countries paused their AstraZeneca vaccination programme.
“India’s own committee that looks at adverse effects is seized of this issue. For the last few days, it is tracking the information that is available to us in a very systematic manner and again I assure you that we have no signal of concern in this regard. Therefore, clearly our vaccination programme with Covishield will go on with full vigour.
“We are mindful of the fact to address this concern, based on the emerging situation. As of today, there is no concern at all with regard to Covishield,” Paul had said last week.
The growing hesitation among citizens over taking the Covishield jab has caused a major dent in India’s Covid-19 vaccination drive which is being touted as the world’s biggest.
Fears among citizens worsened after the Centre recently revised the time window within which the 2nd dose of Covishield vaccine can be taken, asking all states and Union Territories to administer it between 4-8 weeks instead of 4-6 weeks.
“The time interval between two doses of the Covishield vaccine revised from 4-6 weeks to 4-8 weeks to ensure better protection. Any delay beyond the eighth week will leave the beneficiary vulnerable,” the ministry said today adding that protection from the virus is ‘greater if the vaccine is taken between 6-8 weeks’.
Read all the Latest News, Breaking News and Coronavirus News here


















Comments
0 comment