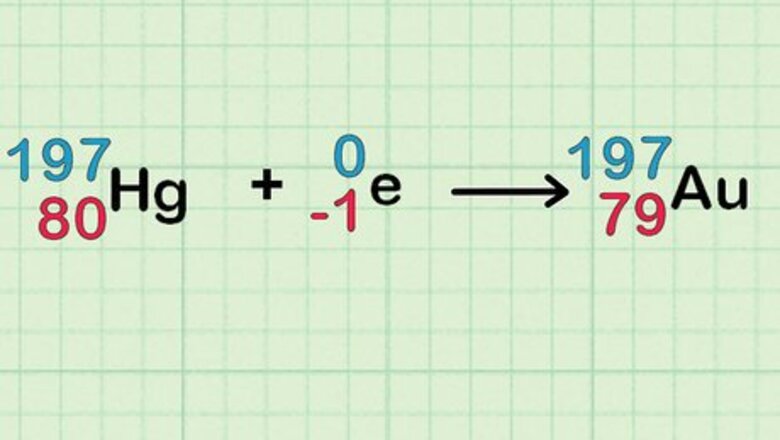
views
Gather the equipment. To turn mercury into gold, you will need the following: Mercury (duh....). Get as much as you can, because it's not very efficient. A neutron source. Ideally you need a particle accelerator (or a nuclear reactor). A neutron moderator. Water (yes, the stuff out of a tap) works pretty well. Neutron shielding, reflecting and collimating equipment Nitric acid
Configure the equipment. The neutrons from the source need to pass through the collimator (a thing that makes them go in straight lines) and the moderator (something that slows them down so that they react better) and finally into the mercury. Set up the neutron reflectors around the mercury (so that any escaping neutrons are reflected back) and then surround the whole thing with shielding.
Fire up the accelerator. Ideally, you want to hit the mercury with a short, very intense burst of neutron radiation.
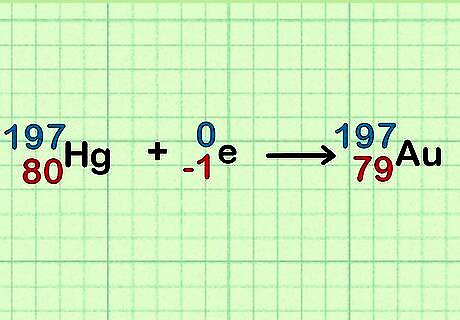
Understand the science! Here's what's happening: Ordinary mercury contains seven isotopes: Hg-196, Hg-198, Hg-199, Hg-200, Hg-201, Hg-202 and Hg-204. When it absorbs a neutron, Hg-196 becomes Hg-197. Hg-197 decays into gold. As a by-product, Hg-202 and Hg-204 become Hg-203 and Hg-205, which decay into thallium. Other isotopes change into each other and remain mercury.
Wait. After the neutron burst, the reaction that produces gold has a half-life of 64.14 hours. If you wait this long, half of the Hg-197 will have become gold. Proton plus Hg-200 is one unfavorable (due to high positive nuclei charge and repulsion charge), yet with a possible alternate pathway.
Treat the mixture with nitric acid. After the reaction, the mixture should largely contain mercury, with some thallium isotopes, and gold. Nitric acid will dissolve mercury and thallium, but not gold.
Filter off the mercury and thallium nitrates. You should be left with gold. And now for the bad news: Though Hg-196, the isotope is readily converted to gold as Hg-196 readily absorbs neutrons, it only comprises 0.15% of natural mercury left. Assuming perfect conversion to Hg-197, then after one half-life (half of the Hg-197 decays), a kilogram of mercury will give you... 0.73 grams of gold. That's right, you get less than three-quarters of a gram of gold per kilo of mercury. To further increase gold yields and make better use of mercury, you can try to hit a isotope specific mix of Hg-198, Hg-199 (together 27% of all mercury) with high energy protons, kicking out neutrons. Yet this is an extremely inefficient process. Keep doing it until finally to converted to Hg-197, then wait just as above for decay to Au-197 or gold. Repeat nitrate steps

















Comments
0 comment