views
Identifying the Basics
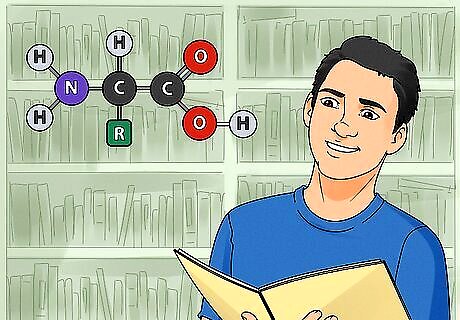
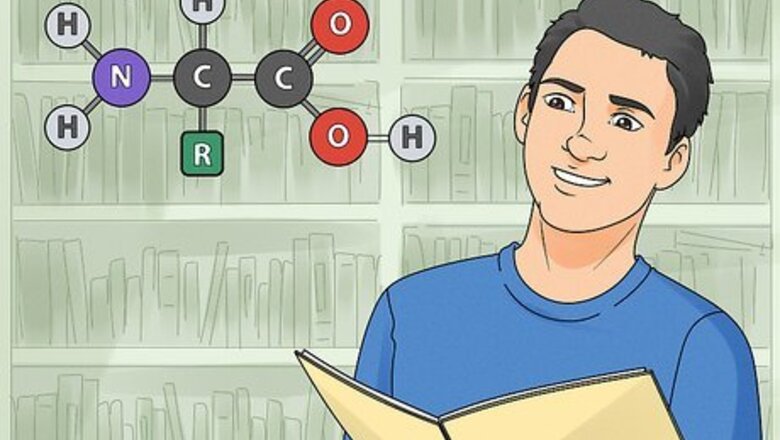
Memorize the structure of amino acids. Amino acids are the building blocks of all proteins. Memorizing the structure and properties of all 20 amino acids is an essential aspect of biochemistry. Know their single and triple letter abbreviations to quickly recognize them while studying. Making flashcards is a great way to memorize the structures of amino acids. Learn the amino acids in 5 groups of 4. Memorize essential properties such as acidic (negatively charged) versus basic (positively charged) and polar versus hydrophobic. Draw their structures over and over until you have them committed to memory. Fortunately, the amino acids have similar structures. They each contain a basic amino group (−NH2), an acidic carboxyl group (−COOH), and a hydrogen group (-H). They are distinguished by an organic R group (or side chain), which determines their function and is unique to each amino acid.
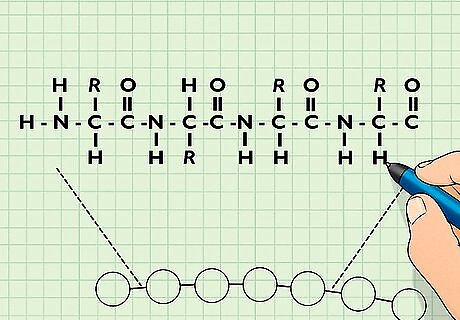
Recognize protein structures. Proteins are composed of chains of amino acids. Recognizing the various levels of protein structures and being able to draw the important ones (alpha helices and beta sheets) are fundamental concepts in biochemistry. There are four levels of protein structure: Primary structure is the linear arrangement of amino acids. They are held together by peptide bonds in a polypeptide chain. Secondary structure makes up the sections of proteins that fold into alpha helices and beta sheets, which are driven by hydrogen bonding. Tertiary structure is the three-dimensional structure resulting from the interactions between amino acids, usually driven by disulfide bonds, hydrogen bonding, and hydrophobic interactions. It is the physiological shape of the protein. The tertiary structure of many proteins is still unknown. Quaternary structure results from multiple separate proteins interacting together to form one larger, single protein. They often contain subunits and are globular.
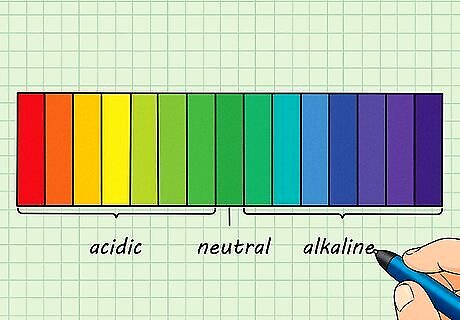
Understand the pH scale. The pH of a solution is a measure of acidity. This is related to the amount of hydrogen and hydroxide ions present in the solution. An acidic solution has more hydrogen ions present in the solution and fewer hydroxide ions. The opposite is true for basic solutions: more hydroxide ions, fewer hydrogen ions. Acids are hydrogen ion (H) donors and have a pH < 7. Bases are hydrogen ion (H) acceptors and have a pH > 7.
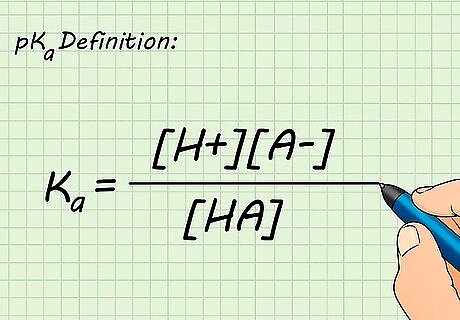
Define the pKa of a solution. The Ka of a solution is used to predict the extent of acid dissociation or how readily the acid gives up its hydrogen ions. It is defined by the equation Ka = [H][A]/[HA]. The Ka of most solutions can be located in a table in your textbook or online. The pKa is defined as the negative log of the Ka. Strong acids dissociate completely and have very small pKas. Weak acids dissociate incompletely and have higher pKas.
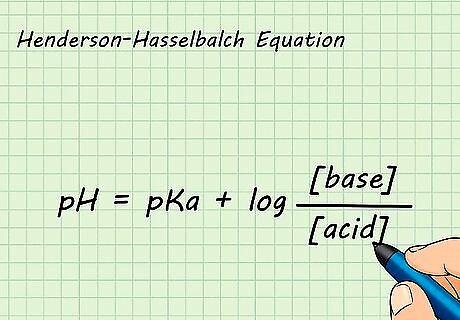
Relate pH and pKa using the Henderson-Hasselbalch Equation. The Henderson-Hasselbalch Equation is used to prepare buffers for solutions in the laboratory. It is also used in acid-base reactions to find the equilibrium pH. The equation states that pH = pKa + log [base]/[acid]. The pKa of a solution is equal to the pH of the solution when the concentrations of the acid and the base are the same. A buffer is a solution that resists changes in pH when small amounts of acidic or basic solutions are added to it. They are important for keeping solutions at a stable pH. Buffers are also important in biological systems, such as keeping the human body at a pH of 7.4.
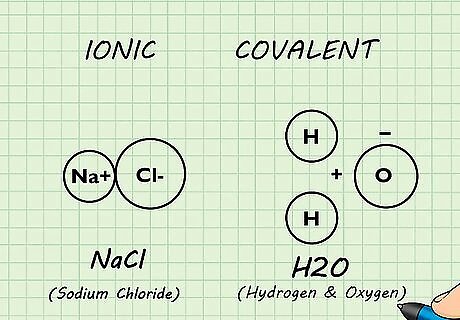
Recognize ionic and covalent bonds. An ionic bond forms between atoms when one or more electrons is removed from one atom and donated to the other atom. The resulting positive and negatively charged ions then attract each other. Covalent bonds form when two atoms share pairs of electrons. Other forces such as hydrogen bonds (attractive forces between hydrogen atoms and highly electronegative molecules) are also important. The type of bond formed between atoms determines some of the properties that molecule will have.
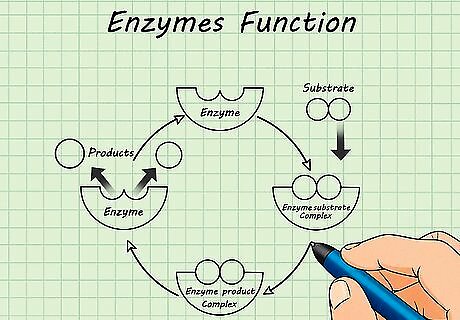
Learn about enzymes. Enzymes are an important class of proteins in the body that are used to catalyze (increase the speed of) biochemical reactions by lowering activation energy. Almost every biochemical reaction in the body is catalyzed by a specific type of enzyme; therefore, the investigation of the mechanism of enzymatic function is a major subject in biochemistry. It is investigated mostly from the kinetic point of view. Enzyme inhibition is used pharmacologically to treat many types of diseases that affect the body. Enzymes do not change or get used up in reactions, so they can perform many rounds of catalysis.
Memorizing Metabolic Pathways

Read and study the figures of the pathway. There are a number of essential metabolic pathways you will have to memorize when taking biochemistry: glycolysis, oxidative phosphorylation, the citric acid cycle (Krebs Cycle), the electron transport chain, and photosynthesis, to name a few. Read the associated text in your book and study the figure detailing the process of the pathway. It’s likely that you will need to be able to draw the entire cycle on a test.
Learn one pathway at a time. If you try to study all of the pathways at once, you will confuse them and won’t have a strong foundation for any of them. Focus on learning one pathway and review that pathway for a few days before you move onto the next one. Once you have learned one, don’t let it fade away. Redraw it often to make sure you keep it fresh in your mind. Take online quizzes or have a friend quiz you so the metabolic pathways stay fresh in your mind.
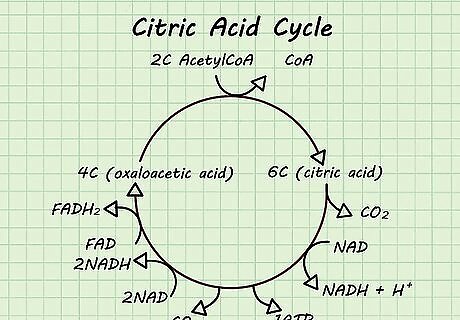
Draw the basic pathway. When you first start learning, start with the basic pathway. Some pathways are a cycle that continue (the citric acid cycle) while other pathways are a linear process (glycolysis). Start learning by memorizing the shape of the pathway, where the pathway starts, what gets broken down, and what gets synthesized. For each cycle, you will have starting molecules such as NADH, ADP, or glucose, and end products such as ATP and glycogen. Memorize these general pieces first.
Add in the cofactors and metabolites. Now get more specific with the pathway. Metabolites are intermediate molecules that get formed during the process, but then get used up as the reaction continues. There are also cofactors that are required for or help to increase the rate of the reaction. Avoid memorizing for the sake of memorizing. Learn how each intermediate transforms into the next so that you have an actual understanding of the process instead of just rote memorization.
Draw in the necessary enzymes. The final step to memorizing the pathway is adding in the enzymes necessary to carry out the reaction. Learning the pathway in pieces like this will make it less overwhelming to start with. Once you learn all the names of the enzymes, you will have the whole pathway finished. You should now be able to easily write out every protein, metabolite, and molecule involved in the metabolic pathway. Make sure you know which steps of the pathway are irreversible and why (if applicable).

Review the pathways frequently. This type of information needs to be reviewed and redrawn on a weekly basis or else you will forget it. Take time each day to review a different pathway, and be sure you know where in the body it takes place. By the end of the week you will have reviewed them all and then you can start all over the next week. When test time comes around, you won’t have to worry about learning all of the metabolic pathways because you will have already have them memorized.
Studying Basics

Read the textbook. Reading the textbook for any course you take is essential for studying the subject. Before class, read and review the material that will be covered that day. Take notes on what you read and you will be better prepared for class. Be sure to read for comprehension. At the end of each section, summarize the material in your notes. Try answering some of the questions at the end of the chapter to check your understanding of the concepts.

Study the figures of the textbook. The figures of your textbook are very detailed and help to visualize what the text is telling you. Oftentimes, it is much easier to understand a concept by looking at a picture of it than just reading words. Redraw important figures in your notes to go back and study later.

Color code your notes. There are many complicated processes in biochemistry. Develop and use a color coding system for your notes. Maybe write your notes based on difficulty with one color representing really complicated concepts and another color, concepts you easily remember and understand. Use a system that works for you. Don’t just copy your friend’s notes and hope it makes you better at studying. Avoid overdoing it. Using too many different colors will turn your notes into a rainbow, but will not be as useful.

Ask questions. While you are reading the textbook, write down questions on statements or concepts that are confusing. Ask questions during lecture as well. Don’t be afraid to raise your hand. If you have a question, it’s likely that other people in the class have the same question. Seek out your teacher to ask questions that may not have been answered during class time.
Make flashcards. There are a lot of vocabulary words associated with biochemistry that you may not have seen before, and many of the words are similar. Learning these words and the differences between them in the beginning will help you understand the concepts later that build upon this vocabulary. Write out paper flashcards or make digital flashcards that you can carry with you on your phone. Whenever you have downtime, take out your flashcards and flip through them.
















Comments
0 comment