views
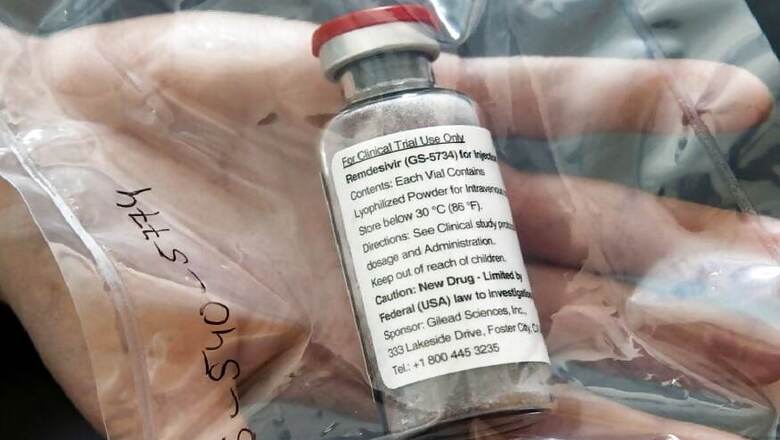
The Union health ministry on Friday revised the dosage of anti-viral drug remdesivir to be administered to coronavirus patients in the moderate stage of illness from the earlier six days to five days as it issued an updated 'Clinical Management Protocols for COVID-19'.
The drug, administered in the form of injection, should be given at a dose of 200 mg on day one followed by 100 mg daily for four days (total five days), the new treatment protocols stated. The Health Ministry on June 13 had allowed the use of remdesivir for restricted emergency use in moderate cases under "investigational therapies".
"Under emergency use authorisation, remdesivir may be considered for patients in moderate stage requiring oxygen support," the document stated. It is not recommended for those with severe renal impairment and high level of liver enzymes, pregnant and lactating women, and those below 12 years, it said.
The ministry also okayed off-label application of tocilizumab, a drug that modifies the immune system or its functioning, and convalescent plasma for treating COVID-19 patients in the moderate stage of illness as "investigational therapies".
It also recommended hydroxychloroquine for patients during the early course of the disease and not for critically-ill patients.
On June 27, the ministry had included an inexpensive, widely used steroid dexamethasone in treatment protocols for COVID-19 patients in the moderate to severe stages of their illness among other therapeutic measures.
The ministry advised use of dexamethasone, which is already used in a wide range of conditions for its anti-inflammatory and immunosuppressant effects, as an alternative choice to methylprednisolone for managing moderate to severe cases of coronavirus infection.
India's COVID-19 cases soared by over 20,000 in a day for the first time taking the country's total tally to 6,25,544 on Friday while the death toll climbed to 18,213 with 379 new fatalities, according to the Union Health Ministry data updated at 8 am.
















Comments
0 comment